To Break a Chemical Bond Energy Must Be
To break a chemical bond energy must be. As a chemical bond is formed either by transfer of electrons or sharing of electrons in order to break the forces of attraction among atoms energy must be absorbed.

When Does The Breaking Of Chemical Bonds Release Energy Science Questions With Surprising Answers
What occurs when an atom of chlorine and an atom of hydrogen become a molecule of hydrogen chloride.
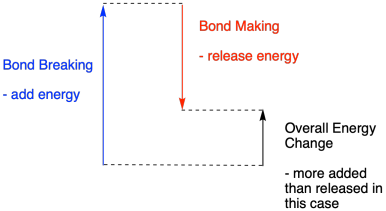
. Less energy is released than absorbed during the reaction. Select the correct answer below. The energy of the OH bond is 467 kJmol.
Select the correct answer below. 16 What is the most likely electronegativity value for a metallic element. H-H would absorb the least amount of energy to be broken.
14 To break a chemical bond energy must be 1 absorbed 2 destroyed 3 produced 4 released. Added released released Question. In order to break a chemical bond energy must be _____________ because it takes _____________ to rip two atoms apart and overcome the attractive force bw them.
It takes roughly 100 kcal of energy to break 1 mol of CH bonds so we speak of the bond energy of a CH bond as being about 100 kcalmol. A chemical bond is formed and energy is released. When a bond is form potential energy ____________ and the stability of the substance formed _____________.
Energy must be _____ to break chemical bonds and _____ when chemical bonds form. Absorbed 2 H2 O2 H2O2 HH OO HOOH The energy of the HH bond is 432 kJmol. Breaking bonds absorbs energy.
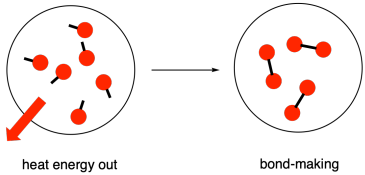
Breaking chemical bonds absorbs energy while making new bonds releases energy with the overall. B Energy is absorbed as bonds are formed. After all molecules dont spontaneously break.
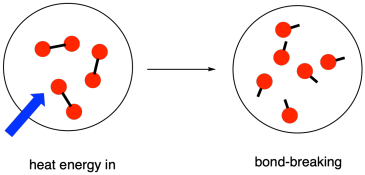
A Energy is absorbed as bonds are broken. 2-5 nitrogen will have 5 dots. The stronger a bond is the more.
Energy must be _____ to break chemical bonds and _____ when chemical bonds form. Added released released added added. The amount needed is called the bond energy.
To break a chemical bond energy must be absorbed. 15 Which Lewis electron-dot diagram represents a nitrogen atom in the ground state. Energy must be _____ to break chemical bonds and _____ when chemical bonds form.
Energy is released and new bonds are formed. Select the correct answer below. For example when is the last time you saw a pile of wood spontaneously burst into flames or a bucket of water turn into hydrogen and oxygen.
Energy must be _____ to break chemical bonds and _____ when chemical bonds form. Energy is _____________ when a bond is formed and must be __________ in order to break a bond. Which atom attains a stable valence electron configuration by bonding with a chemical bond.
To break a chemical bond energy must be. Energy is never destroyed it converts from one form to another. D Energy is released as bonds are formed.
The bond energy for H-H is 436 kJ while the bond energy for H-Cl is 431 kJ. C Energy is released as bonds are broken. What Happens When Chemical Bonds Break And New Bonds FormChemical reactions make and break the chemical bonds between molecules resulting in new materials as the products of the chemical reaction.
To break a chemical bond energy must be a produced b absorbed c released d destroyed. OO 3 H2 CO2 CH2O2. You have to put energy into a molecule to break its chemical bonds.
Added released released added added added released released. The larger the bond energy the weaker the connection. Which bond is the strongest.
The energy of the OO bond is 495 kJmol. Breaking Bonds Requires Energy. Energy is produced or released when a chemical bond is formed.
A CC bond has an approximate bond energy of 80 kcalmol while a CC has a bond energy of about 145 kcalmol. Bond dissociation energy kJmol The energy required to break the bond between two atoms. Select the correct answer below.
In order to break a bond energy must be. A chemical bond results when two nuclei have a simultaneous attraction for a nucleons b neutrons c protons d electrons. The energy of the OO bond is 146 kJmol.
H-Cl is the stronger bond. When a chemical bond is broken energy is.

Question Video Calculating The F F Bond Energy From The Molar Bond Energy Of F Using Avogadro S Number Nagwa

Bond Energy Definition Equation Calculations Study Com

Lesson Explainer Bond Energy Nagwa

When Chemical Bonds Are Broken Energy Is Lisbdnet Com
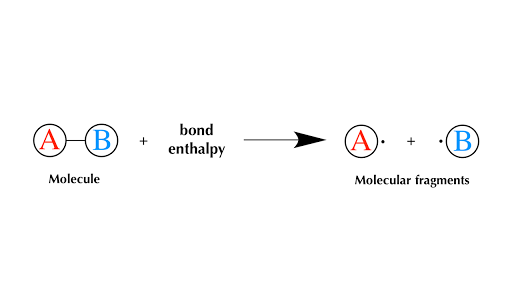
Bond Enthalpies Article Enthalpy Khan Academy
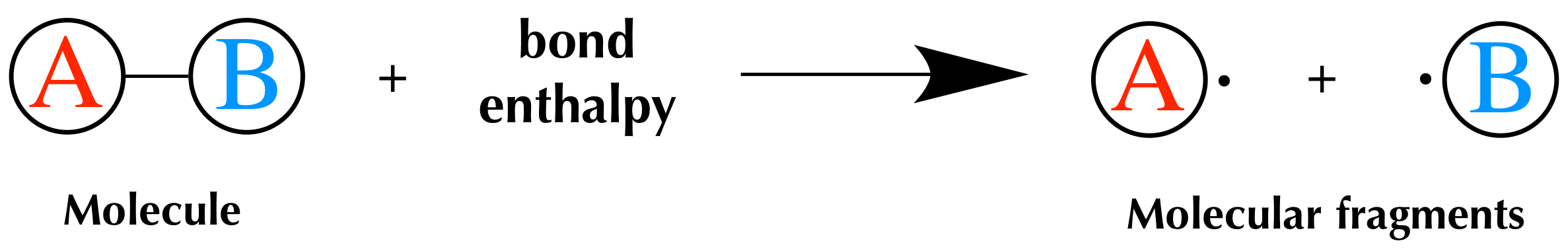
Bond Enthalpies Article Enthalpy Khan Academy
Is The Activation Energy And The Energy To Break Bonds The Same I Think It S Not But Can Somebody Explain This To Me Quora

7 5 Strengths Of Ionic And Covalent Bonds Chemistry
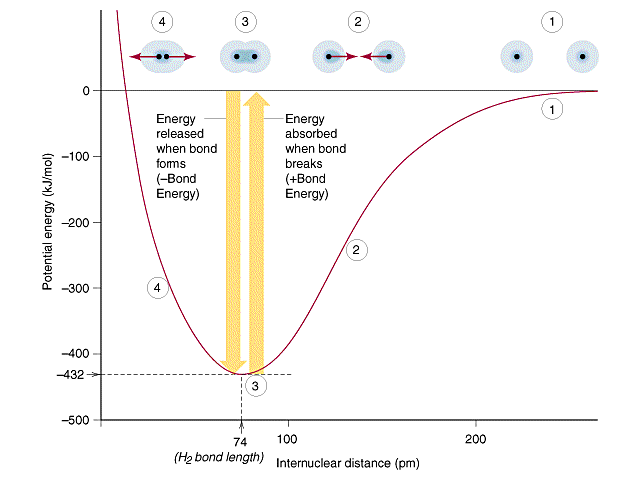
8 9 Covalent Bond Properties Order Length And Energy Chemistry Libretexts

Dublin Schools Lesson Bond Energy

Lesson Explainer Bond Energy Nagwa

Bond Energy Definition Equation Video Lesson Transcript Study Com

Question Video Definition Of Hydrogen Molecules Bond Energy Nagwa
Comments
Post a Comment